Our lab is investigating the use of CRISPR/Cas9 based genome editing to replace genes in airway (and other) stem cells for therapeutic applications
We are working to develop genetic therapies for cystic fibrosis (CF) using CRISPR/Cas9 based gene editing
For background information on genetic therapies please scroll to the second half of the page
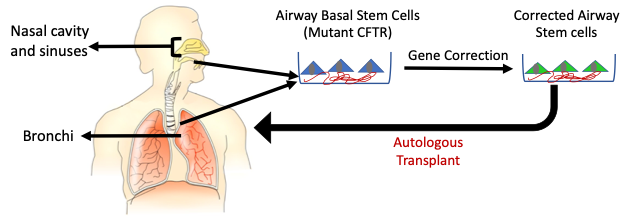
Engineered airway stem cell therapy to treat cystic fibrosis
Cystic fibrosis (CF) is a common genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CF is a life-limiting disease that affects ~70-200,000 people world-wide that affects many organs including the lungs. People with CF experience lung failure caused by repeated bacterial infections. Replacing the CFTR gene in airway stem cells that regenerate the lung epithelium may durably treat CF. In previous work, it was proposed that the concept could be tested in the sinus epithelium which has a similar composition as the lower airway epithelium. Our lab is using CRISPR/Cas9 technology to develop an autologous gene edited airway stem cell therapy to treat CF lung disease.
Engineered airway stem cell therapies to treat other diseases
The sinus epithelium is readily accessible by minimally invasive endoscopic procedures. It is possible to engineer the upper airway basal stem cells from the sinuses using CRISPR/Cas9 technology. We are interested in using engineered airway cells to treat genetic diseases other than CF. In addition, we are interested in testing their use for delivery of protein therapeutics to treat other non-genetic disorders.
In vivo genome editing
CF is a systemic disorder affecting the lungs, pancreas, liver and reproductive organs. We are interested in developing methods to directly gene edit stem cells in organs in vivo using CRISPR/Cas9.
Genetics and DNA (Late 19th and Early 20th Centuries)
It had been known for centuries that traits are passed down from one generation to the next in all kingdoms of life (plants, animals, and humans). However, the exact biological mechanisms were not known. Gregor Mendel, from the 19th century, is widely recognized for characterizing the inheritance of traits in pea plants. This prompted the scientific community to search for the factor/factors that were responsible for passing down these traits. It was first proposed that proteins carried genetic information responsible for different traits. A series of experiments investigating the transformation of a non-virulent bacterial strain into a virulent one culminated in the discovery that the substance passing these traits was composed of deoxyribonucleic acids (DNA). The double helical structure of DNA was described by Drs. Watson and Crick using data from Dr. Rosalind Franklin’s group in 1953.
How do we replace genes in people?
(1980s-Now)
Introducing new genes into humans is tricky because the therapy must be effective but without any adverse side effects. Early studies used retroviruses that randomly insert DNA sequences into our genome. An early trial attempted to replace the gene adenosine deaminase (ADA) in the T-cells of a patient suffering from an immunodeficiency disorder. But the effects were transient. It was later realized that such gene replacement needs to be performed in stem cells that regenerate our organs through our lifetime in order to provide a durable benefit. Subsequently, ADA was successfully replaced in blood stem cells using retroviruses and resulted in long-term benefit. However, semi-random insertion of genes poses the risk of cancer. It is also challenging if the expression of the gene needs to be regulated precisely. In parallel, several groups of use a non-integrating virus called adeno-associated virus (AAV) to replace genes in the retina, nervous system and liver to treat genetic disorders. This technology provides long-term benefit for diseases affecting organs with slow or no cell division. However, new methods are needed to diseases affecting organs such as the hematopoietic system, intestines, skin and lungs which have high rates of cell turn over.
Recombinant DNA and the Idea of Genetic Therapies (1940s-1970s)
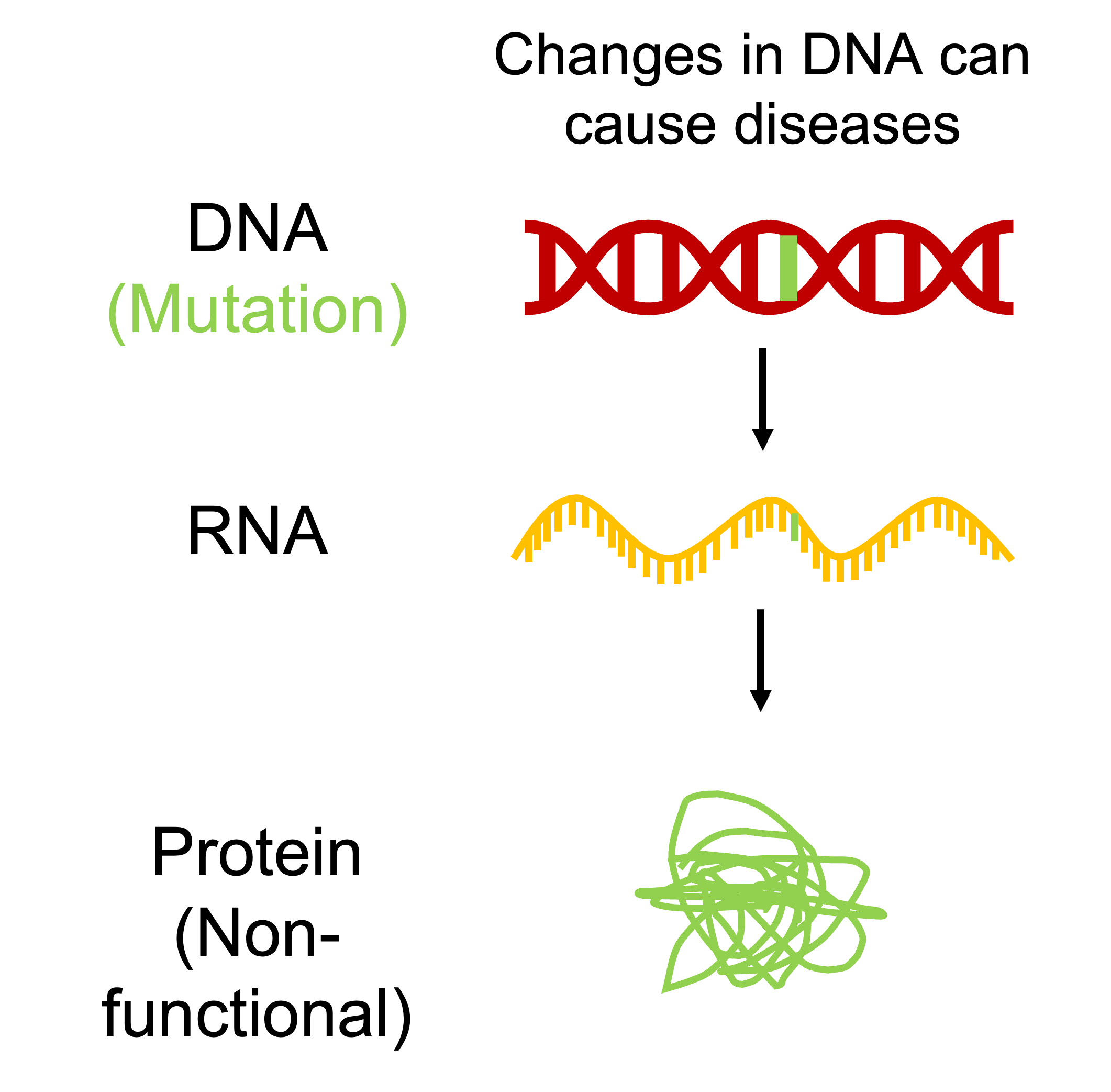
After the description of the structure of DNA, it was discovered that DNA contained information to produce proteins that are responsible for most cellular functions and ultimately determine our attributes. For example, a single DNA base pair change in the gene coding for the beta subunit of the protein hemoglobin results is the cause for sickle cell disease. In the 1970s, scientists demonstrated that it was possible to cut and past DNA molecules derived from different organisms to generate new DNA (recombinant DNA) in vitro. This technology was subsequently used to enable production of the human protein insulin (used to treat diabetes) in bacteria. These discoveries prompted the idea that perhaps we could treat genetic disorders such as sickle cell disease and cystic fibrosis by replacing erroneous genes present in these individuals.
How do we replace genes in people?
(1980s-Now)
Introducing new genes into humans is tricky because the therapy must be effective but without any adverse side effects. Early studies used retroviruses that randomly insert DNA sequences into our genome. An early trial attempted to replace the gene adenosine deaminase (ADA) in the T-cells of a patient suffering from an immunodeficiency disorder. But the effects were transient. It was later realized that such gene replacement needs to be performed in stem cells that regenerate our organs through our lifetime in order to provide a durable benefit. Subsequently, ADA was successfully replaced in blood stem cells using retroviruses and resulted in long-term benefit. However, semi-random insertion of genes poses the risk of cancer. It is also challenging if the expression of the gene needs to be regulated precisely. In parallel, several groups of use a non-integrating virus called adeno-associated virus (AAV) to replace genes in the retina, nervous system and liver to treat genetic disorders. This technology provides long-term benefit for diseases affecting organs with slow or no cell division. However, new methods are needed to diseases affecting organs such as the hematopoietic system, intestines, skin and lungs which have high rates of cell turn over.
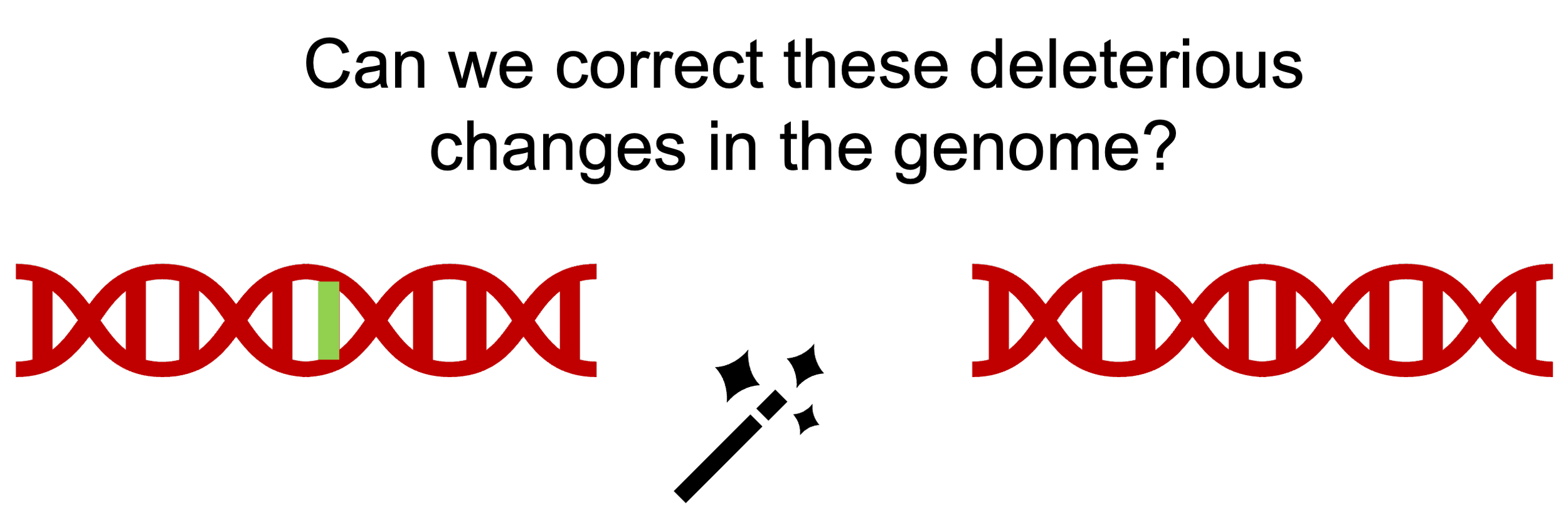
Genome Editing for Precise Gene Replacement
(1980s - Now).
In the 1980s, it was shown that introduction of foreign DNA with sequences homologous (identical) to a target in the genome can enable the insertion of new DNA sequences in the region at a low efficiency. Subsequent studies showed that inducing a break in the DNA can enhance this process of gene insertion significantly. but there was no method to introduce breaks in specific regions within the genome. In the 1990s, artificial nuclease enzymes that can cleave DNA at precise locations were engineered by fusing DNA binding proteins with nucleases (zinc finger nucleases or ZFN). In the early 2000s, it was shown that break generated in a target locus in the genome using ZFNs could be used to increase the efficiency of gene insertion into that target locus. This process was still inefficient for practical use. In 2012, a bacterial enzyme called Cas9 was reported that could be targeted to target locations in the genome using a guide RNA. Cas9 was then used to insert genes into the genome of hematopoietic stem cells with high efficiency. Such gene edited stem cells are being tested for treating sickle cell disease and thalassemia in clinical trials. But how do we apply this technology to other organs for which we don’t have methods to transplant stem cells? Our lab is pioneering the use of this technology to treat airway diseases starting with cystic fibrosis.